-
 Mary Wang
Hi there! Welcome to my shop. Let me know if you have any questions.
Mary Wang
Hi there! Welcome to my shop. Let me know if you have any questions.
Your message has exceeded the limit.

TOC Cleaning Validation Kits Instructions: Step-by-Step Guide
2025-08-08 17:39:05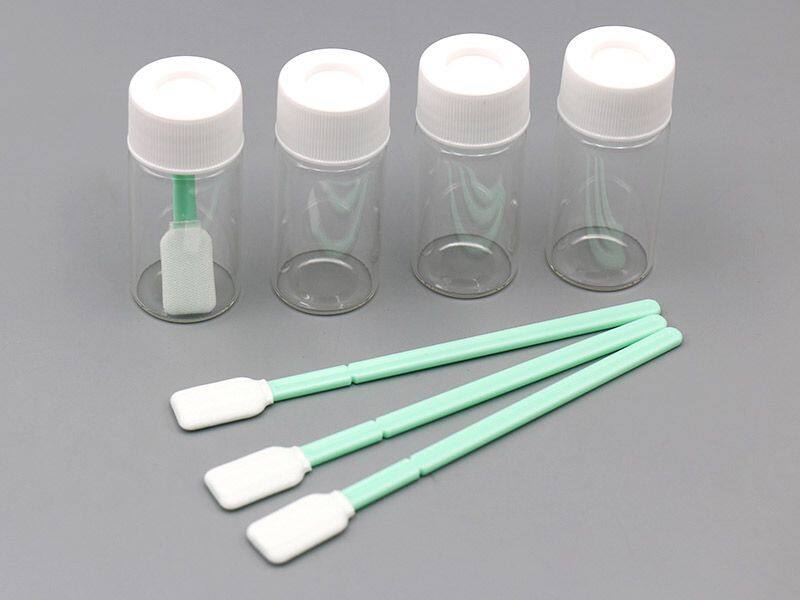
Total Organic Carbon (TOC) cleaning validation is a critical process in pharmaceutical and biotechnology industries. Proper use of a TOC cleaning validation kits ensures your facility meets regulatory standards and maintains product quality. This guide provides clear, step-by-step instructions for using your TOC cleaning validation kits effectively.

Before beginning, always consult your written Standard Operating Procedures (SOPs) for specific sampling instructions relevant to your site and application. Ensure you have all necessary materials, including the TOC cleaning validation kits, gloves, and sample labels.
Step-by-Step InstructionsFollow these steps for accurate sample collection using your TOC cleaning validation kits:
1. Dip the Swab in SolutionOpen the sampling solution provided with your kits.
Dip the swab into the solution. This moistens the swab, aiding in the efficient collection of potential contaminants.
Use the moistened swab to sample the designated surface area.
Follow your SOPs for the correct technique and sampling pattern to ensure thorough coverage.
Carefully open the sample vial without touching the interior, preventing any risk of contamination.
Align the swab head with the notch at the top of the vial.
Apply gentle but firm pressure to snap off the swab head.
Allow the swab head to fall into the vial without touching it to maintain sample integrity.
Securely replace the cap on the vial to seal the sample.
Immediately label the vial with all required information, such as sample location, date, time, and operator initials.
Proper labeling ensures traceability and compliance.
Wear gloves and use aseptic technique throughout the process.
Avoid touching the swab head at any time to prevent contamination.
Handle all materials carefully to maintain the integrity of your sample.
Follow your facility’s SOPs for documentation and chain of custody.
Choosing the right kit, like the MEIDIKE GENE TOC Cleaning Validation Kits, helps you achieve accurate, regulatory-compliant results every time. For more information or to request a quote for the MEIDIKE GENE TOC Cleaning Validation Kits, contact our team today and take the next step toward streamlined, effective cleaning validation!

Tags: TOC Cleaning Validation Kits, TOC Kits, Cleaning Validation Swab


